Bharat Biotech’s nasal COVID-19 vaccine to cost Rs 800, Currently available on CoWIN website, it will be out in the market by Year-end.
Bharat Biotech’s iNCOVACC, India’s first intranasal Covid-19 vaccine approved for booster doses. They are priced at Rs 800 per dose for the market and at Rs 325 for government hospitals. The Hyderabad-based company said the vaccine is available on the government’s CoWIN website. It will be available in the market for those above 18.
Bharat Biotech’s needle-free vaccine – BBV154 – received approval from the Drugs Controller General of India in November for restricted use in emergency for those above 18. The vaccine has been approved for mix-and-match usage (or heterologous vaccination), wherein a beneficiary may be administered shots of different Covid vaccines. Earlier this month, it was approved as heterologous booster dose.
The vaccine, iNCOVACC, is the world’s first intranasal vaccine for Covid to receive approval for the preliminary 2-dose schedule, the Hyderabad-based company had said. The pharma giant’s executive chairman Dr Krishna Ella said, “We have achieved the goals we set for ourselves during this pandemic. We have developed COVAXIN and iNCOVACC, two COVID vaccines from two different platforms with two other delivery systems.”
“The vectored intranasal delivery platform gives us the capability for rapid product development, scale-up, easy and painless immunization during public health emergencies and pandemics,” Ella said. Two separate and simultaneous clinical trials were conducted to evaluate BBV154 as a primary dose (2-dose) schedule; and a heterologous booster dose for subjects who have previously received two doses of the two commonly administered covid vaccines in India.
The trials for the primary dose were conducted among 3,100 subjects in 14 trial sites across the country, while the heterologous booster dose was done at nine trial sites among 875 subjects. iNCOVACC is stable at 2-8°C for easy storage and distribution. Bharat Biotech has established extensive manufacturing capabilities at multiple sites across India, including in Gujarat, Karnataka, Maharashtra and Telangana.
A Needle-free vaccine
Bharat Biotech’s needle-free vaccine – BBV154 – received approval from the Drugs Controller General of India in November for restricted use in emergency for those above 18. The vaccine has been approved for mix-and-match usage (or heterologous vaccination), wherein a beneficiary[divider style=”double” top=”20″ bottom=”20″] may be administered shots of different Covid vaccines.
Key Attributes by Bharat Biotech :
- An intranasal vaccine stimulates a broad immune response – neutralizing IgG, mucosal IgA, and T cell responses.
- Immune responses at the site of infection (in the nasal mucosa) – essential for blocking both infection and transmission of COVID-19.
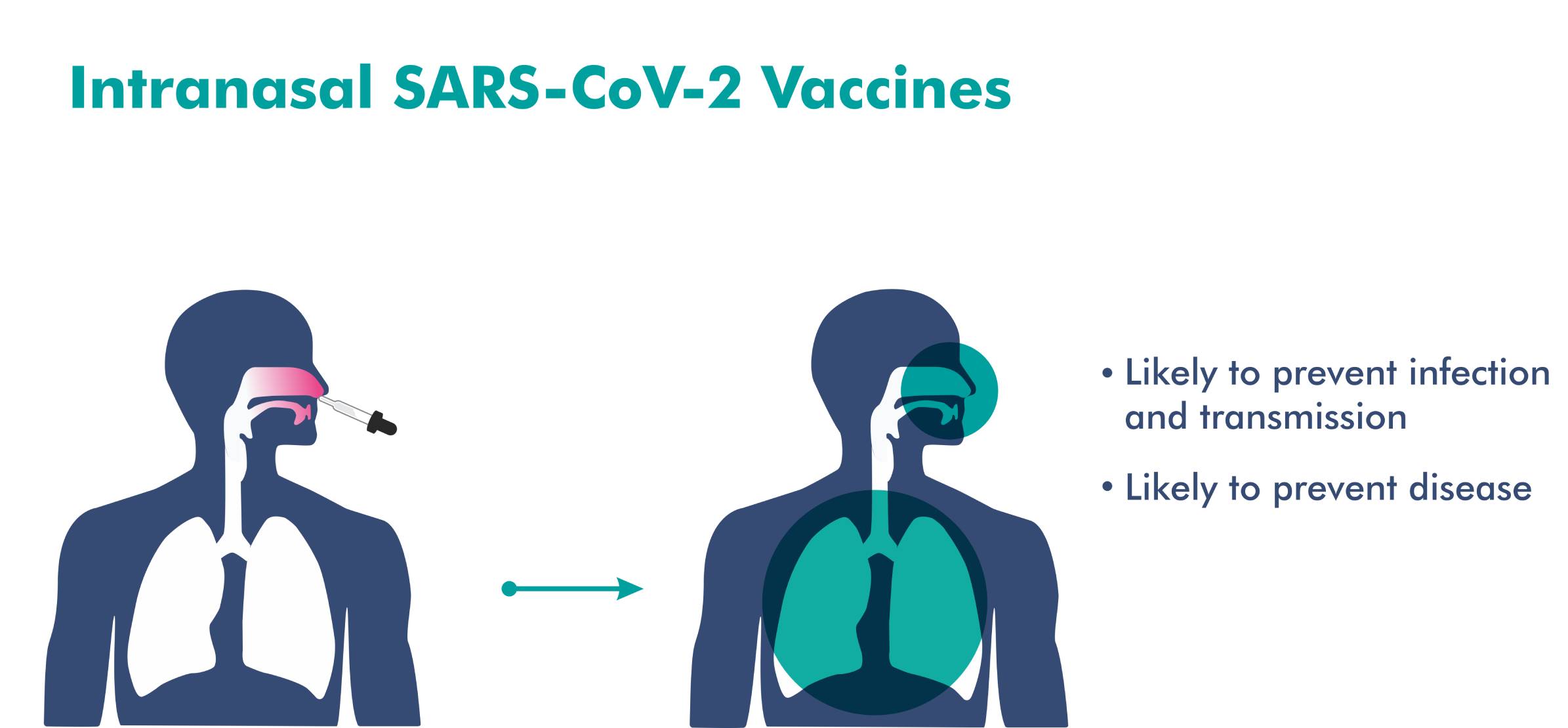
- The nasal route has excellent potential for vaccination due to the organized immune systems of the nasal mucosa.
- Non-invasive, Needle-free.
- Ease of administration – does not require trained health care workers.
- Elimination of needle-associated risks (injuries and infections).
- High compliance.
- Scalable manufacturing – able to meet global demand.